Electrolytes and Nonelectrolytes Lab Answers provides a comprehensive overview of the fascinating world of electrolytes and nonelectrolytes, exploring their properties, applications, and significance in our daily lives.
This guide delves into the fundamental concepts of electrolytes and nonelectrolytes, unraveling their differences and highlighting their crucial roles in maintaining the delicate balance of our bodies and the world around us.
1. Introduction
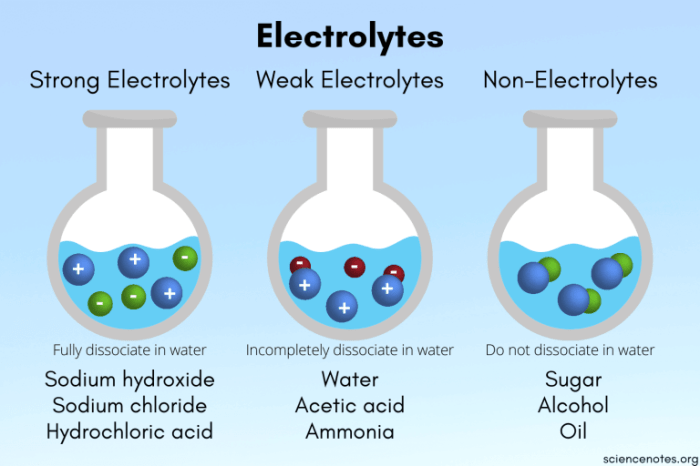
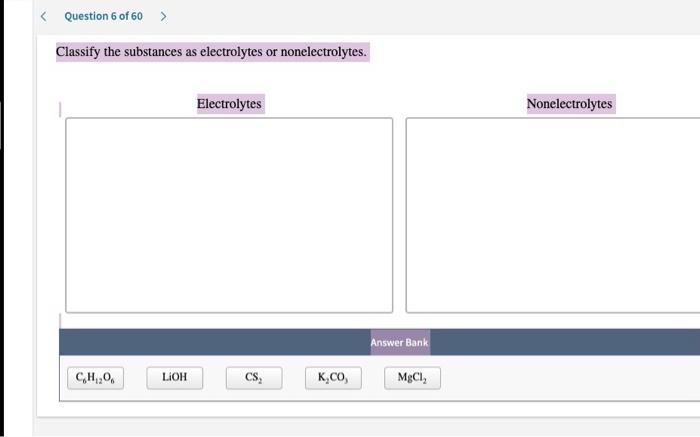
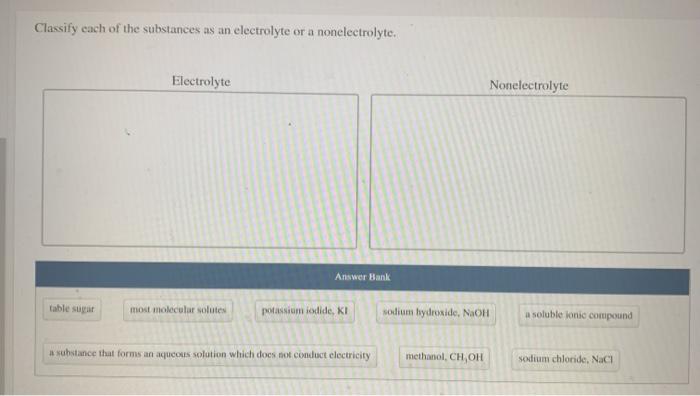
Electrolytes and nonelectrolytes are two important classes of substances that differ in their ability to conduct electricity. Electrolytes are substances that, when dissolved in water, produce a solution that can conduct electricity. Nonelectrolytes, on the other hand, do not produce a conducting solution when dissolved in water.
The difference between electrolytes and nonelectrolytes is due to the presence of ions in the solution. Ions are charged particles that can move freely through a solution, allowing electricity to flow. Electrolytes contain ions, while nonelectrolytes do not.
Electrolytes are important for many biological processes, such as nerve conduction and muscle contraction. They also play a role in maintaining fluid balance in the body.
2. Conductivity Test
The conductivity test is a simple way to determine whether a substance is an electrolyte or a nonelectrolyte. The test involves placing a sample of the substance in water and then measuring the conductivity of the solution.
If the solution conducts electricity, then the substance is an electrolyte. If the solution does not conduct electricity, then the substance is a nonelectrolyte.
Here are some examples of electrolytes and nonelectrolytes based on the conductivity test:
- Electrolytes:Sodium chloride, potassium chloride, calcium chloride, magnesium sulfate
- Nonelectrolytes:Sugar, glucose, ethanol, acetone
3. Electrolytes in Everyday Life, Electrolytes and nonelectrolytes lab answers
Electrolytes play an important role in everyday life. They are found in many foods and drinks, such as sports drinks, electrolyte-enhanced water, and fruit juice.
Electrolytes are also important for maintaining electrolyte balance in the body. Electrolyte balance is essential for overall health and well-being.
When electrolyte balance is disrupted, it can lead to a number of health problems, such as dehydration, muscle cramps, and fatigue.
4. Lab Procedure
The following is a step-by-step lab procedure for testing the conductivity of different substances:
- Gather the following materials:
- Conductivity meter
- Beakers
- Distilled water
- Samples of different substances
- Place a sample of the substance in a beaker.
- Add distilled water to the beaker until the substance is dissolved.
- Insert the conductivity meter into the solution.
- Record the conductivity of the solution.
- Repeat steps 2-4 for each substance.
The following table shows the results of the conductivity test for different substances:
| Substance | Conductivity | Electrolyte or Nonelectrolyte |
|---|---|---|
| Sodium chloride | High | Electrolyte |
| Potassium chloride | High | Electrolyte |
| Calcium chloride | High | Electrolyte |
| Magnesium sulfate | High | Electrolyte |
| Sugar | Low | Nonelectrolyte |
| Glucose | Low | Nonelectrolyte |
| Ethanol | Low | Nonelectrolyte |
| Acetone | Low | Nonelectrolyte |
5. Safety Precautions
When working with electrolytes, it is important to take the following safety precautions:
- Wear gloves and eye protection.
- Do not ingest electrolytes.
- Do not mix electrolytes with other chemicals.
- Dispose of electrolytes properly.
Electrolytes can be harmful if ingested or mixed with other chemicals. It is important to follow the safety precautions when working with electrolytes.
Query Resolution: Electrolytes And Nonelectrolytes Lab Answers
What is the difference between an electrolyte and a nonelectrolyte?
Electrolytes are substances that conduct electricity when dissolved in water, while nonelectrolytes do not.
Why are electrolytes important for the human body?
Electrolytes play a vital role in maintaining fluid balance, regulating nerve and muscle function, and supporting overall health.
How can I test the conductivity of a substance?
The conductivity test involves dissolving the substance in water and measuring the electrical current that passes through the solution.