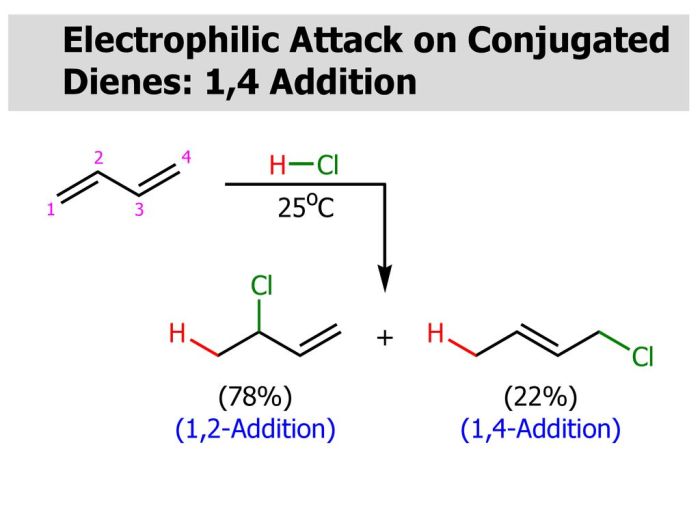
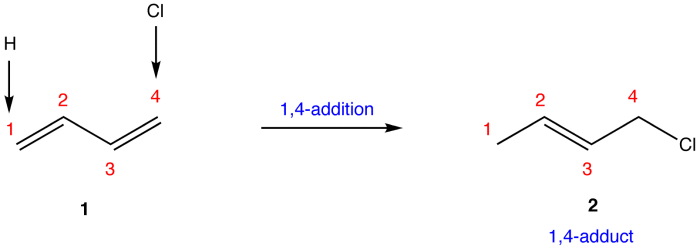
Electrophilic attack on conjugated dienes is a fundamental reaction in organic chemistry, offering a versatile approach for the synthesis of complex molecules. This reaction involves the addition of an electrophile to a conjugated diene system, leading to the formation of a new carbon-carbon bond.
The regioselectivity and stereoselectivity of this reaction are influenced by various factors, making it a subject of ongoing research and applications.
This comprehensive guide explores the electrophilic attack on conjugated dienes, providing a detailed overview of its mechanism, regioselectivity, stereoselectivity, reaction conditions, applications, and computational studies. By delving into these aspects, we aim to enhance your understanding of this important reaction and its significance in organic synthesis.
Electrophilic Attack on Conjugated Dienes
Electrophilic attack on conjugated dienes is a fundamental reaction in organic chemistry. It involves the addition of an electrophile to a conjugated diene, resulting in the formation of a new carbon-carbon bond. This reaction is highly regio- and stereoselective, and it has numerous applications in organic synthesis.
Regioselectivity and Stereoselectivity, Electrophilic attack on conjugated dienes
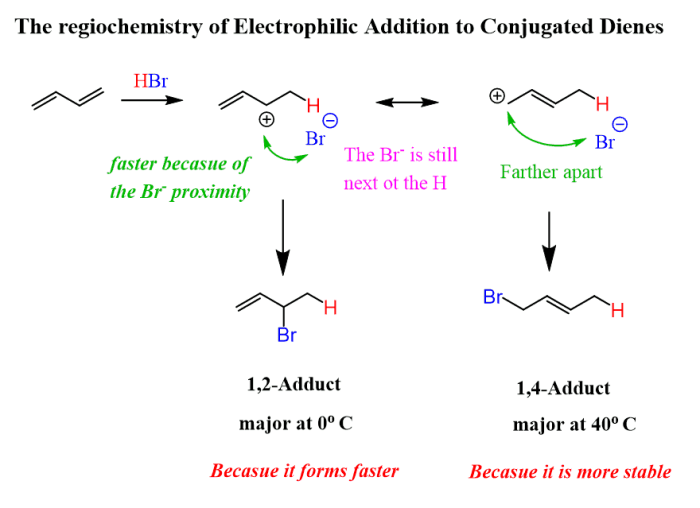
The regioselectivity of electrophilic attack on conjugated dienes is determined by the stability of the carbocation intermediate that is formed. The more stable the carbocation, the more likely it is to be formed. The stereoselectivity of the reaction is determined by the orientation of the electrophile relative to the diene.
The electrophile can add to either the top or bottom face of the diene, resulting in the formation of two different stereoisomers.
Reaction Conditions
The typical reaction conditions for electrophilic attack on conjugated dienes involve the use of a strong electrophile, such as HBr or Br2, in a nonpolar solvent, such as dichloromethane. The reaction is typically carried out at room temperature, but it can also be carried out at higher or lower temperatures.
The rate of the reaction is increased by the addition of a Lewis acid catalyst, such as AlCl3 or BF3.
Applications
Electrophilic attack on conjugated dienes is a versatile reaction that has numerous applications in organic synthesis. It can be used to synthesize a variety of compounds, including alkenes, aldehydes, and ketones. The reaction is also used in the synthesis of natural products and pharmaceuticals.
Computational Studies
Computational studies have played an important role in the understanding of electrophilic attack on conjugated dienes. These studies have helped to elucidate the reaction mechanism and to predict the regioselectivity and stereoselectivity of the reaction. Computational methods have also been used to design new catalysts for the reaction.
Q&A: Electrophilic Attack On Conjugated Dienes
What is the driving force behind electrophilic attack on conjugated dienes?
The electrophilic attack is driven by the formation of a new carbon-carbon bond between the electrophile and the diene system. The conjugated diene system provides a higher electron density, making it more susceptible to electrophilic attack.
How can the regioselectivity of the electrophilic attack be controlled?
The regioselectivity of the reaction can be controlled by factors such as the nature of the electrophile, the substitution pattern of the diene, and the reaction conditions. Electron-withdrawing substituents on the diene tend to direct the attack to the more substituted carbon.
What are the applications of electrophilic attack on conjugated dienes?
Electrophilic attack on conjugated dienes is widely used in organic synthesis for the construction of various cyclic and acyclic compounds. It is particularly useful for the synthesis of natural products and pharmaceuticals.